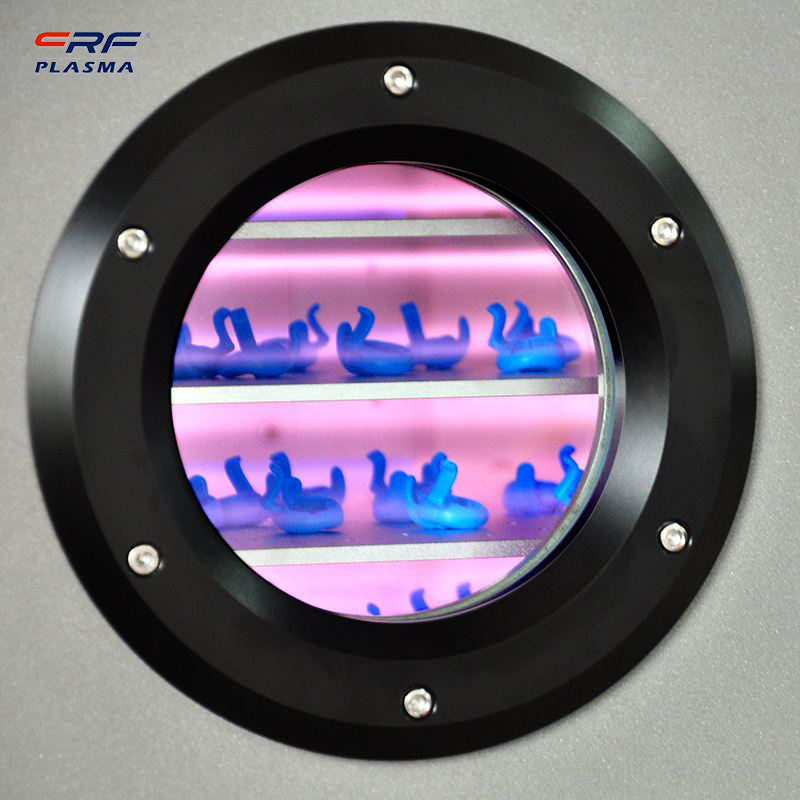
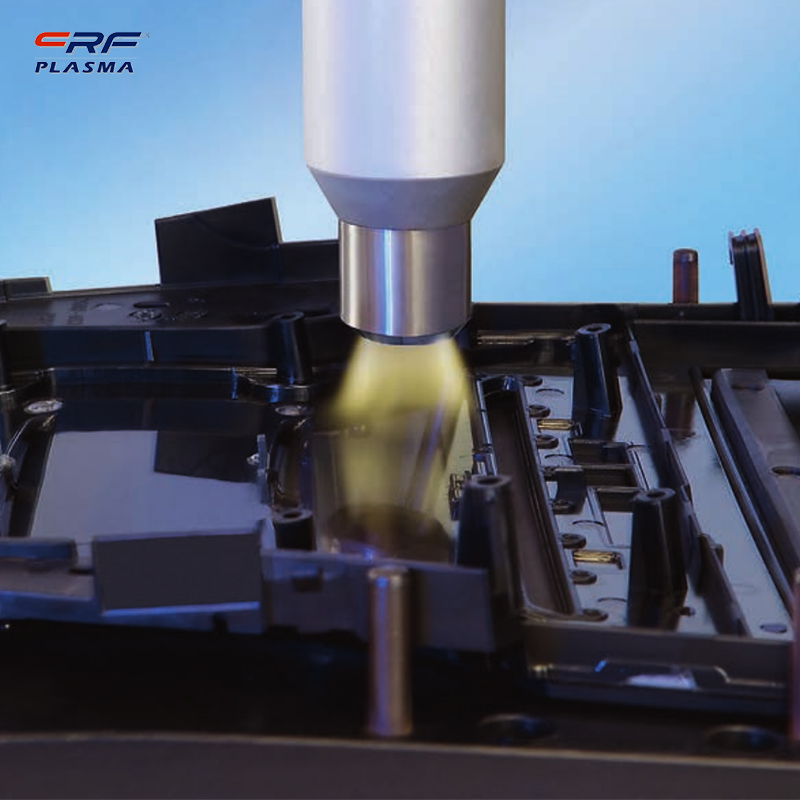
Welcome to Shenzhen Sing Fung Intelligent Manufacturing Co., Ltd.
E-mail:shaobo@sfi-crf.com
News
Low-temperature plasma technology to solve the use of three methods environmental engineering wastewater
The use of low-temperature plasma technology to treat environmental engineering wastewater can achieve a better treatment effect under the joint action of high-energy electron radiation, ozone oxidation, ultraviolet decomposition and other methods.
High-energy electron action.
Low-temperature plasma technology generates a large number of high-energy electrons in the wastewater treatment process, which convert energy into internal energy of the substrate molecules through collisions with atoms and molecules in the wastewater, and active the wastewater through several processes such as excitation, decomposition and ionization.
New compounds are formed by breaking down the molecular bonds in the wastewater and reacting with active factors such as free oxygen and ozone. Finally, Z eventually converts toxic substances into non-toxic substances and degrades pollutants in the raw wastewater.
Ozone oxidation.
In the process of wastewater treatment, ozone acts as a strong oxidizing agent to chemically combine harmful substances and form certain intermediate products, which reduces the toxicity and harmful substances of the original sewage, and after several reflections, eventually decomposes the organic substances of the polluted substances into carbon dioxide and water. For inorganic substances, certain oxides can be formed for removal.
Ultraviolet decomposition effect.
Using low-temperature plasma technology, UV irradiation can decompose harmful substances alone, or jointly with ozone. Separate decomposition effect is mainly harmful molecular substances by absorbing photons into the excited state, through the absorption of energy to break the molecular bonds of molecules, and then react with the free substances in the water to generate new compounds released.
Oxidation by UV light and ozone can treat difficult to degrade substances simultaneously with better effect. Refractory organic substances and pesticides can be broken down quickly.


Study of plasma working environment on improving the hydrophilicity of PITFE material surface
Study of plasma working environment on improving the hydrophilicity of PITFE material surface:
Polytetrafluoroethylene (PIFE) has been widely used in industrial applications because of its unique properties such as excellent chemical stability, dielectric properties, very low coefficient of dynamic friction, better machinability and flame retardancy. Diaphragm paper, also known as polyethylene (PE) film, its role in lithium-ion batteries is to isolate the positive and negative materials, and the quality of the diaphragm paper directly affects the safety performance and capacity of the battery, so the choice of high-quality diaphragm paper has been a must for battery manufacturers. Biocompatible silicone rubber and polyester as a new thermoplastic engineering plastics have a wide range of uses, but due to the poor surface wettability of such polymer materials and other defects, limiting its application in the medical, health and other special industrial technology fields.
Most of the surfaces of new polymeric materials are hydrophobic, which limits their adhesive and other applications. Plasma surface modification is a very forward-looking method to optimize the structure and properties of the material surface by plasma. The main methods of surface activation are chemical etching, optical radiation, plasma treatment, ion implantation, and surface graft polymerization. Plasma surface modification is to optimize the structure of the material surface by discharging plasma, which has become a common material surface modification method in industry because of its specific environmental and cost advantages. pife, pe, silicone rubber polyester, strip-like samples, can be treated with continuous drive plasma. When the power is the same, the modification effect of plasma is Ar+H, N2, O2 in descending order. increasing the power is instead detrimental to the improvement of surface hydrophilicity of PIFE samples, which is due to the fact that at high power, there are significantly more high-energy particles in the plasma, which strengthen the impact on the material surface and can make some reactive groups on the surface inactive, thus reducing the introduction of reactive groups.
The pressure has no significant effect on the contact angle when the discharge voltage is stronger than 10 Pa as well as less than 50 Pa. However, when the air pressure is greater than 50 Pa, the contact angle increases instead, which may be due to the high air pressure that makes it difficult for the gas to be completely ionized, thus affecting the modification of the PTFE surface. Plasma gives the material new surface properties, but the effect of plasma surface treatment has the problem of timeliness, with the placement time and show certain changes, with the extension of time, the surface contact angle will gradually increase. The reason for the decay of the time-dependent wettability of the plasma-treated but ungrafted surface may be multifaceted, which may be due to the failure of the newly introduced hydrophilic groups to dive into the surface of the material after a period of time; it may also be due to the cross-linking chemical reactions on the surface, thus decreasing the hydrophilicity of the material surface. Therefore, in order to prevent the failure of the plasma-treated surface, grafting and bonding must be carried out within a specified period of time to maintain and make full use of the modification effect.
Plasma contains a large number of electrons, ions, excited atoms, molecules and radicals and other reactive particles, these reactive particles and polymer materials interact to oxidation, reduction, cleavage, cross-linking and polymerization of various physical and chemical reactions on the surface of the material, thus optimizing the surface properties of the material, increasing the surface hygroscopicity (or hydrophobicity), dyeability, adhesion, antistatic properties and bio compatibility, etc. Plasma achieves surface modification of polymers such as polytetrafluoroethylene, PE battery separators, silicone rubber, and polyesters. Plasma working conditions have a significant effect on improving the hydrophilicity of PITFE material surfaces. Plasma treatment introduces a large number of polar groups on the surface of the material, which is responsible for the improvement of its hydrophilicity.
Translated with www.DeepL.com/Translator (free version)


plasma cleaning machine-surface treatment equipment-CRF plasma machine-Sing Fung Intelligent Manufacturing
According to the temperature of heavy particles in plasma, plasma can be divided into two categories, hot plasma and cold plasma. At 3×103K-3×104K, the thermodynamic equilibrium is basically reached, with a uniform thermodynamic equilibrium temperature, and the plasma state and the plasma state can be determined by Maxwell's thermodynamic equilibrium velocity distribution, Boltzmann particle energy distribution, Shaha equation and other methods. parameter. Thermal plasma has high energy density and is mainly used for material synthesis, spheroidization, densification and coating protection.
In low temperature plasma, the temperature of heavy particles is only room temperature, while the temperature of electrons can reach thousands of degrees, so it is far away from the thermodynamic equilibrium state, such as glow discharge belongs to low temperature plasma. Cold plasma is mainly used for plasma etching, deposition and plasma surface decoration. The temperature of electric washing is a concern of many users. During electric washing, the flame of electric washing looks similar to the general flame. However, if the electric washing equipment adopts an intermediate frequency power supply, it has high power and strong energy, and the temperature without water cooling is also very high. If the washing material is not temperature resistant, pay attention to the temperature.
The power supply of the plasma cleaning machine is commonly used 13.56KHz radio frequency power supply, which produces high plasma density, soft energy, and low temperature. , the intermediate frequency power is 40KHz, contrary to the radio frequency power supply, the plasma density is not high, but the power is large, the energy is high, and the high power is tens of KW. The temperature of the vacuum plasma cleaner discharge RF cleaner is similar to the usual indoor temperature. Of course, if the vacuum plasma cleaner is used all day, it still needs to add water to the cooling system.
The average temperature of the plasma jet is between 200 and 250 degrees Celsius. If the distance and speed can be set correctly, the surface temperature can reach 70-80℃. This process is suitable for cleaning various standard products (metals, ceramics, glass, plastics, elastomers, etc.). Generally, a jet plasma cleaning device is used, and it can also be applied to a plasma cleaning device on an assembly line. Due to the continuous operation of the assembly line , the material stays under the nozzle for a long time. Nitrogen can also be used for some jet plasma cleaning due to its ability to lower the plasma temperature. It should be noted that there is another product in the plasma cleaning machine called corona. In fact, corona is also a classification of plasma cleaning machines. The washing temperature of the corona machine is generally higher, and the washing temperature of the corona machine is similar to that of the jet plasma. Sometimes materials that are not resistant to high temperature are also cleaned with nitrogen. In fact, there is no need to worry too much about temperature. The current plasma cleaning machine can adjust the temperature well, so that the material cleaning can achieve the desired effect


The modification of fiber surface by plasma technology has also received extensive attention
The modification of fiber surface by plasma technology has also received extensive attention
Since the 21st century, with the rapid development of science and technology and modern industry, in order to meet the development needs of various industries, new materials with various functions have emerged one after another. At the same time, this has also prompted the development and progress of various surface modification technologies.
Plasma surface modification refers to exposing the material to non-polymer gas plasma, and bombarding the surface of the material with the plasma to change the surface structure of the material, thereby realizing activation modification. In general, the surface modification of the functional layer is very thin (several nanometers to hundreds of nanometers), which will not affect the overall performance of the material; after modification, the surface of the material can be hydrophilic, anti-wear, decorative, coloring properties, printing, adhesion, antistatic and other functions.
The modification of fiber surface by plasma technology has also received extensive attention. Plasma treatment of the carbon fiber surface can not only improve the adhesion, but also ensure that the tensile strength of the fiber does not decrease. In addition, plasma treatment can also eliminate surface microcracks of carbon fibers, reduce stress concentration, and improve the tensile strength of the fibers themselves. Plasma treatment of Kevlar fiber and aramid fiber has the same effect. PET fiber is widely used, but its dyeing, hygroscopic and antifouling properties are poor. After plasma treatment, polar groups are introduced on the surface to generate free radicals and cross-linked layers, which effectively improve various properties.
During the production process of industrial components such as electronic components and auto parts, various contaminants will form on the surface due to cross-contamination, natural oxidation, flux, etc. quality, reducing the reliability and yield of finished products. Plasma treatment processes the surface of the workpiece through chemical or physical action, and the reactive gas is ionized to generate highly reactive reactive ions, which chemically react with surface contaminants for cleaning. The reaction gas needs to be selected according to the chemical composition of the contaminant. The plasma cleaning based on chemical reaction is fast, has good selectivity, and has better cleaning effect on organic pollutants. Argon gas is commonly used for plasma cleaning in which the surface reaction is mainly based on physical action, which does not produce oxidation by-products, and the etching effect is anisotropic. In general, in the process of plasma surface modification, chemical reaction and physical action coexist, so as to obtain better selectivity, uniformity and directionality.
Due to the development direction of precision and miniaturization in the industrial field, plasma surface modification technology will have more and more important applications in the semiconductor industry, chip industry, aerospace and other high-tech industries due to its advantages of fine cleaning and non-destructive modification. value.


Today, I will tell you how the plasma etches the LDPE film.
Plasma modification mainly has the following advantages:
①It is a dry process, which meets the current needs of energy saving and environmental protection;
②There are no special requirements for the materials to be processed, and it has universal applicability;
③ The processing time is short, only a few seconds to a few minutes;
④ Only modify the surface layer of the material without causing damage to the substrate itself. Therefore, the plasma technology has better application effect than the traditional modification technology. Plasma is a non-condensed system produced by complete or partial ionization of the gaseous state. The so-called "ionization" means that at least one electron is detached from an atom or molecule, thereby converting the atom or molecule into a positively charged ion. The system includes atoms, molecules, ionic excited states and metastable states. The number of positive and negative charges in the system is equal, and the system is macroscopically neutral. The application of plasma technology in material science is particularly significant. The development of new materials is to modify their surfaces through plasma technology in order to achieve higher performance, which is an important means of research and development of new materials. In the process of modifying the material surface by plasma, the original chemical bond on the material surface is broken to form a new chemical bond usually by hitting the material surface. With the exception of ions, most of the ions in the plasma have energies higher than chemical bond energies. This suggests that plasma can break chemical bonds on the surface of materials and form new ones.
The surface modification of plasma treatment is to expose the material to the plasma of non-polymeric gas, and bombard the surface of the material with the plasma to change the polymer structure, so as to achieve the purpose of modifying the surface of the polymer material.
Plasma treatment is mainly aimed at inert gases. Organic polymer materials are treated with non-polymeric inorganic gases such as oxygen, nitrogen, hydrogen, and argon, and then contact with air, which will introduce functional groups on the surface to form cross-linked structural layers or generate free radicals. In general, the hydrophilicity of the surface is greatly improved after plasma treatment of the surface.
The crystallinity and timeliness of PET film after surface modification were studied. After treatment under dielectric barrier discharge conditions, the water contact angle of the film decreases with the increase of energy density, and the biaxially stretched film with the highest crystallinity has the smallest contact angle. The etching effect of air plasma on the LDPE film is the most obvious, so the surface morphology change is the most prominent. Under the best conditions, the peel strength after bonding is significantly increased compared with that before treatment. This is because the active groups generated by air plasma interact with the surface of LDPE to increase active particles and attract oxygen-containing groups. In addition, due to the timeliness of plasma treatment, the next process should be entered immediately after treatment.
After the PTFE is treated by plasma jet, the hydrophilic property of the material surface is enhanced. It was found from the SEM pictures that the treated PTFE surface produced dense micron-sized particles, which increased the surface roughness, and the density and roughness of these particles increased with the prolongation of the treatment time. This is because the cleavage of C-F bonds on the surface of PTFE introduces oxygen-containing groups.
After the plasma modification treatment, the hydrophilicity and surface roughness of the material surface are greatly improved, and there is a linear increase trend with the working pressure. The increased nitrogen content after plasma treatment improves the biocompatibility of the polycarbonate.


Today, Chengfeng Zhizao will analyze the precautions for the power supply of low-temperature plasma cleaning equipment
The following will tell you a few things that basically all types of low-temperature plasma cleaning equipment should pay attention to when using it. Now with the development of science and technology, there are more and more types of low-temperature plasma cleaning equipment. It is only necessary to understand the instructions for use. Some low-temperature plasma equipment also require operators to receive on-the-job training. With the development of science and technology, many low-temperature plasma equipment has begun to have a self-cleaning function, and the daily maintenance of the equipment has become more concise.
In the experiment, when we want to clean, activate, corrode, deposit or polymerize the surfaces of various materials, low-temperature plasma processing equipment is a necessity, and as a high-precision equipment often used in laboratories, the price is naturally high. If Improper operation when using low-temperature plasma equipment is likely to cause damage to the equipment or affect the operation results, so today Chengfeng Zhizao will tell you about the precautions for the use of low-temperature plasma cleaning equipment.
First, the low-temperature plasma cleaning equipment requires a power supply with a frequency of 220V AC and a frequency of 50HZ during operation. The connection and inspection of the power supply should be done before turning on the equipment. After the equipment is running, it should emit a smooth running sound. And usually, there will be a green indicator light on to show that the equipment is running normally. If the yellow fault light is on, stop the operation for scheduled inspection.
Second, when using Chengfeng Zhizhi low temperature plasma cleaning equipment, special attention should be paid to the red warning light. Usually, the red light will be always on when there is a problem with the operation of the equipment or the jitter frequency is too fast. At this time, you should press the reset button immediately to observe If the equipment is still abnormal, the operation of the equipment should be stopped, and then the troubleshooting should be carried out to avoid damage to the equipment.
Third, when using low-temperature plasma cleaning equipment, it should be cleaned regularly, and when cleaning, the power must be disconnected first, and then the vacuum chamber and electric control box must be opened, and attention should be paid to cleaning according to the specifications required in the manual. The vacuum chambers of many low-temperature plasma equipment are external toroidal electrodes, so it is not easy to cause contamination in the chamber.
I hope the above explanation can help you, and you are welcome to inquire about this issue, and you can contact us if you need it.


Previous page
1
2
…
46
Next page

TEL:0755-3367 3020 / 0755-3367 3019

E-mail:sales-sfi@sfi-crf.com

ADD:Mabao Industrial Zone, Huangpu, Baoan District, Shenzhen