
Welcome to Shenzhen Sing Fung Intelligent Manufacturing Co., Ltd.
E-mail:shaobo@sfi-crf.com
The effect of energy density on the ethane conversion reaction under the combined action of plasma plasma and 10CeO2/Y-Al2O3
- Categories:Industry News
- Author:Plasma cleaning machine-CRF plasma plasma equipment-plasma surface treatment machine manufacturer-chengfeng intelligent manufacturing
- Origin:
- Time of issue:2022-01-29
- Views:
(Summary description)The effect of energy density on the ethane conversion reaction under the combined action of plasma plasma and 10CeO2/Y-Al2O3: Table 3-5 shows the effect of energy density on the ethane conversion reaction under the combined action of plasma plasma and 10CeO2/Y-Al2O3. When the energy density reaches 300 kJ/mol, the plasma reaction is initiated, and the conversion rate of C2H6 and CO6 increases with the increase of the energy density, and the total yield of C2H4 and C2H2 increases accordingly until the energy density reaches 1500 kJ/mol. stability. In a flow plasma reactor, the increase in energy density means that the energy and number of high-energy electrons increase, which will facilitate the reaction of equations (3-26) to (3-29). The relative amount of active species increased. Therefore, the higher the energy density in the plasma reactor, the higher the conversion of C2H6 and CO2. At the same time, the research shows that the increase of energy density is not good for C2H4 or C2H2 selectivity. When the energy density increases from 380 kJ/mol to 1500 kJ/mol, the C2H4 selectivity decreases from 36.0% to 16.2%, and the C2H2 selectivity decreases from 55.4%. fell to 24.2%. Tables 3-5 show the effect of energy density on the gas product C2H4/C2H2 ratio and H2/CO ratio. The C2H4/C2H2 ratio and H2/CO ratio varied in the range of 0.63-0.68 and 2.47-2.91 under different energy densities. Under the action of atmospheric pressure non-equilibrium plasma plasma, C2H6 can undergo oxidative dehydrogenation reaction in CO2 atmosphere to generate C2H2 and C2H4. With CeO2/Y-Al2O3 as the catalyst, CO2 oxidation of CH6 dehydrogenation can occur when the reaction temperature is 973K. The reaction temperature and the ratio of reactant gas have a great influence on the reaction results; when the plasma and the catalyst are jointly activated, CO2 is oxidized to C2H6 conversion. The properties of the catalyst in the reaction have a significant impact on the reaction. The metal oxide catalyst is beneficial to the conversion of ethane to C2H2 and C2H4, and the metal catalyst can increase the percentage of C2H4 in the product.
The effect of energy density on the ethane conversion reaction under the combined action of plasma plasma and 10CeO2/Y-Al2O3
(Summary description)The effect of energy density on the ethane conversion reaction under the combined action of plasma plasma and 10CeO2/Y-Al2O3:
Table 3-5 shows the effect of energy density on the ethane conversion reaction under the combined action of plasma plasma and 10CeO2/Y-Al2O3. When the energy density reaches 300 kJ/mol, the plasma reaction is initiated, and the conversion rate of C2H6 and CO6 increases with the increase of the energy density, and the total yield of C2H4 and C2H2 increases accordingly until the energy density reaches 1500 kJ/mol. stability. In a flow plasma reactor, the increase in energy density means that the energy and number of high-energy electrons increase, which will facilitate the reaction of equations (3-26) to (3-29). The relative amount of active species increased. Therefore, the higher the energy density in the plasma reactor, the higher the conversion of C2H6 and CO2. At the same time, the research shows that the increase of energy density is not good for C2H4 or C2H2 selectivity. When the energy density increases from 380 kJ/mol to 1500 kJ/mol, the C2H4 selectivity decreases from 36.0% to 16.2%, and the C2H2 selectivity decreases from 55.4%. fell to 24.2%. Tables 3-5 show the effect of energy density on the gas product C2H4/C2H2 ratio and H2/CO ratio. The C2H4/C2H2 ratio and H2/CO ratio varied in the range of 0.63-0.68 and 2.47-2.91 under different energy densities.
Under the action of atmospheric pressure non-equilibrium plasma plasma, C2H6 can undergo oxidative dehydrogenation reaction in CO2 atmosphere to generate C2H2 and C2H4. With CeO2/Y-Al2O3 as the catalyst, CO2 oxidation of CH6 dehydrogenation can occur when the reaction temperature is 973K. The reaction temperature and the ratio of reactant gas have a great influence on the reaction results; when the plasma and the catalyst are jointly activated, CO2 is oxidized to C2H6 conversion. The properties of the catalyst in the reaction have a significant impact on the reaction. The metal oxide catalyst is beneficial to the conversion of ethane to C2H2 and C2H4, and the metal catalyst can increase the percentage of C2H4 in the product.
- Categories:Industry News
- Author:Plasma cleaning machine-CRF plasma plasma equipment-plasma surface treatment machine manufacturer-chengfeng intelligent manufacturing
- Origin:
- Time of issue:2022-01-29 15:02
- Views:
The effect of energy density on the ethane conversion reaction under the combined action of plasma plasma and 10CeO2/Y-Al2O3:
Table 3-5 shows the effect of energy density on the ethane conversion reaction under the combined action of plasma plasma and 10CeO2/Y-Al2O3. When the energy density reaches 300 kJ/mol, the plasma reaction is initiated, and the conversion rate of C2H6 and CO6 increases with the increase of the energy density, and the total yield of C2H4 and C2H2 increases accordingly until the energy density reaches 1500 kJ/mol. stability. In a flow plasma reactor, the increase in energy density means that the energy and number of high-energy electrons increase, which will facilitate the reaction of equations (3-26) to (3-29). The relative amount of active species increased. Therefore, the higher the energy density in the plasma reactor, the higher the conversion of C2H6 and CO2. At the same time, the research shows that the increase of energy density is not good for C2H4 or C2H2 selectivity. When the energy density increases from 380 kJ/mol to 1500 kJ/mol, the C2H4 selectivity decreases from 36.0% to 16.2%, and the C2H2 selectivity decreases from 55.4%. fell to 24.2%. Tables 3-5 show the effect of energy density on the gas product C2H4/C2H2 ratio and H2/CO ratio. The C2H4/C2H2 ratio and H2/CO ratio varied in the range of 0.63-0.68 and 2.47-2.91 under different energy densities.
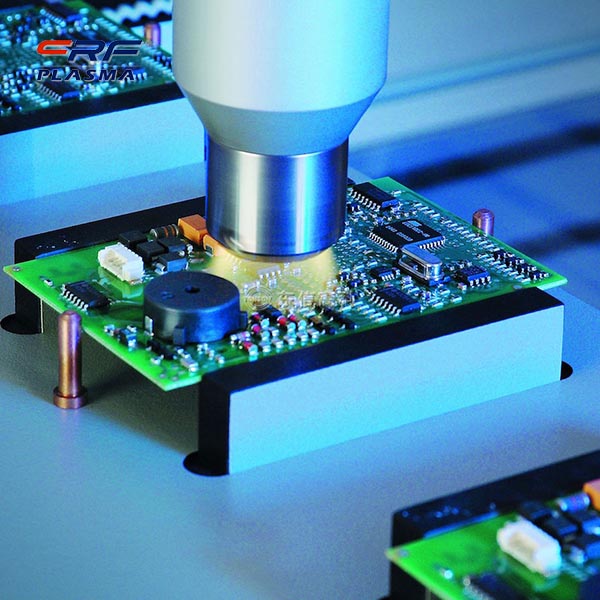 Under the action of atmospheric pressure non-equilibrium plasma plasma, C2H6 can undergo oxidative dehydrogenation reaction in CO2 atmosphere to generate C2H2 and C2H4. With CeO2/Y-Al2O3 as the catalyst, CO2 oxidation of CH6 dehydrogenation can occur when the reaction temperature is 973K. The reaction temperature and the ratio of reactant gas have a great influence on the reaction results; when the plasma and the catalyst are jointly activated, CO2 is oxidized to C2H6 conversion. The properties of the catalyst in the reaction have a significant impact on the reaction. The metal oxide catalyst is beneficial to the conversion of ethane to C2H2 and C2H4, and the metal catalyst can increase the percentage of C2H4 in the product.
Under the action of atmospheric pressure non-equilibrium plasma plasma, C2H6 can undergo oxidative dehydrogenation reaction in CO2 atmosphere to generate C2H2 and C2H4. With CeO2/Y-Al2O3 as the catalyst, CO2 oxidation of CH6 dehydrogenation can occur when the reaction temperature is 973K. The reaction temperature and the ratio of reactant gas have a great influence on the reaction results; when the plasma and the catalyst are jointly activated, CO2 is oxidized to C2H6 conversion. The properties of the catalyst in the reaction have a significant impact on the reaction. The metal oxide catalyst is beneficial to the conversion of ethane to C2H2 and C2H4, and the metal catalyst can increase the percentage of C2H4 in the product.
Scan the QR code to read on your phone

TEL:0755-3367 3020 / 0755-3367 3019

E-mail:sales-sfi@sfi-crf.com

ADD:Mabao Industrial Zone, Huangpu, Baoan District, Shenzhen


















